Generation of OH· at the oil–water interfaces
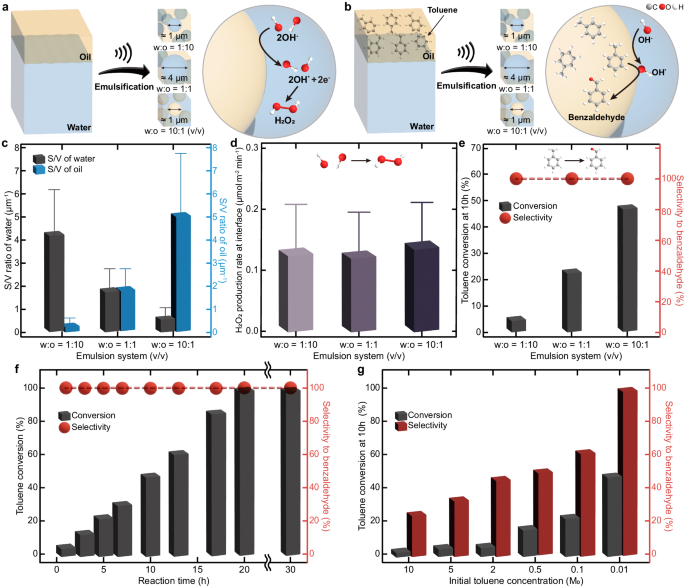
We first investigated the spontaneous generation of OH· at various oil–water interfaces. The intrinsic strong electric field (E \(\approx \, {10}^{9}\) V/m) formed at the oil–water interface7 is sufficient to produce OH· from hydroxide ions (OH–)6,30. We prepared three different oil–water interfaces by emulsifying 1:10, 1:1, and 10:1 (v/v) mixtures of water and hexadecane (Fig. 2a). The created emulsion systems exhibited the ratio of surface area to volume (S/V) ca. 4.3, 1.8, and 0.5 μm−1 for water, and ca. 0.4, 1.8, and 4.8 μm−1 for oil, respectively (Fig. 2c, see Supplementary Fig. 1 for droplet size distribution).
a Schematic illustration of the formation of OH∙ and H2O2 at the oil–water interfaces. Three distinct interfaces were created by emulsifying 1:10, 1:1, and 10:1 (v/v) water and hexadecane mixtures. b Scheme for the oxidation of toluene at the oil–water interfaces. The produced OH∙ at the water-side interface reacts with toluene at the oil-side interface to selectively produce benzaldehyde. c–e S/V ratios of the water and oil phases (c), H2O2 production rate per unit interfacial area (d), and toluene conversion and selectivity for benzaldehyde at 10 h (e) in three different emulsion systems. Each error bar represents the standard deviation of three measurements. f, g The impact of reaction time (0.01 M toluene) (f) and initial toluene concentration (at 10 h) (g) on toluene conversion and selectivity to benzaldehyde: 1 atm oxygen, 25 oC (298 K), water-to-oil ratio of 10:1 (v/v).
The formation of OH· was analyzed by hydrogen peroxide (H2O2) assay with the assumption that the generated OH· readily recombined to form H2O231. We quantified the amount of H2O2 produced in water collected by centrifugation using a spectroscopic method32 (Supplementary Fig. 2). The concentration of H2O2 increased linearly with ultrasound irradiation time, and its production rate was significantly enhanced with an increase in the S/V ratio of water (Supplementary Fig. 3a). However, the efficiency of the oil–water interfaces for OH· generation, as evaluated by H2O2 production rate per unit interfacial area, was quite comparable for all three systems (Fig. 2d). This indicates that, as previously observed6,11,12,17,18,19,33,34,35,36,37, the spontaneous generation of OH· is a general phenomenon of water–hydrophobe interfaces. Other types of oil–water interfaces, including octane, dodecane, benzene, toluene, o-xylene, m-xylene, p-xylene, and 1,2,4-methylbenzene also produced H2O2 effectively (Supplementary Fig. 3b, c), supporting the high chemical activity of various water–hydrophobe interfaces. The decrease in H2O2 production rate induced by the shortening of the carbon chain in the oil phases may be attributed to the reduced interfacial water orientation38, which consequently leads to a reduction in the potential interfacial area occupied by the strong electric field.
Next, we explored the utilization of the created OH· for on water chemistry. As a representative chemical reaction, the free radical polymerization of oil-soluble monomers initiated by the transport of OH· through the interfaces was examined30 (Supplementary Fig. 4a). Dodecyl acrylate (DA) and isodecyl acrylate (IA) were tested as oil-soluble monomers. The 10:1 (v/v) mixtures of water and hexadecane solutions of DA or IA (0.4 M) were prepared and subjected to ultrasound for 2 h. Given that both DA and IA are insoluble in water, the successful polymerization of both monomers (Supplementary Fig. 4b, c) suggests that the generated OH· could effectively interact with oil-dissolved molecules at the interface, thereby facilitating on water chemistry.
Selective oxidation of toluene on water
Selective oxidation of C(sp3)–H bonds is of particular interest in organic synthesis as starting materials for industrial applications, such as pharmaceutical, perfume, dye, and plastics39. Toluene, the simplest alkyl aromatic, can be oxidized to form benzyl alcohol, benzaldehyde, and benzoic acid. These products are commercially synthesized by chlorinating toluene and then saponifying it, which requires harsh reaction conditions and generates toxic waste40. In addition, this procedure requires costly separation processes due to its poor selectivity. Despite the fact that numerous catalysts have been developed to enhance selectivity under mild reaction conditions41,42, conversion efficiency exhibited a marginal improvement in order to achieve high selectivity, and the removal of catalysts is another challenging task. Inspired by the spontaneous generation of OH· at the oil–water interfaces, which can activate C–H bonds in toluene43, we tested their capability in the selective oxidation of toluene under mild, catalyst-free conditions utilizing the oil–water interfaces (Fig. 2b). The 1:10, 1:1, and 10:1 (v/v) mixtures of water and hexadecane solutions containing toluene (0.01 M) were prepared and irradiated with ultrasound to generate an extensive amount of oil–water interfaces. The S/V ratios of oil were ca. 0.4, 1.6, and 5.1 μm−1 for water, and ca. 4.4, 1.6, and 0.5 μm−1 for oil (Supplementary Fig. 5), similar to that of pure hexadecane. Encouragingly, toluene was selectively oxidized to benzaldehyde at 10 h (selectivity >99%) regardless of the different S/V ratios (Fig. 2e, Table 1). The conversion rate was proportional to the S/V ratio of oil, showing that the efficacy of the oil–water interfaces for toluene oxidation was nearly uniform. The negligible toluene oxidations in sonicated bulk solutions (Table 1, entries 9 and 10) and oil-in-water emulsions without ultrasound (Supplementary Fig. 6) support that the existence of the oil–water interfaces generated by ultrasound energy is the determinant factor for the toluene oxidation on water. Moreover, the reduced oxidation rate with increasing concentration of a radical scavenger 4-methoxyphenol44, demonstrates that radical species initiate the toluene oxidation on water (Supplementary Fig. 7).
We further explored the effect of reaction time on the toluene conversion using emulsified 10:1 (v/v) mixtures of water and hexadecane solutions containing 0.01 M toluene (Fig. 2f and Supplementary Table 1). Conversion of toluene linearly increased over time and all toluene was completely oxidized after 20 h. While tiny amounts of hexadecane could be degraded to shorter alkane chains (Supplementary Fig. 8a)35, the impact of these minor products appears to be insignificant, as evidenced by the consistent droplet size (Supplementary Fig. 8b), rate of H2O2 production (Supplementary Fig. 8c), and toluene oxidation rate (Fig. 2f). Regardless of the reaction time, toluene was consistently oxidized to benzaldehyde, and the generated benzaldehyde remained unchanged even after 30 h reactions. In comparison to previous catalytic studies28,29,41,42 (Supplementary Table 2), the oil–water interface exhibited greater benzaldehyde selectivity and the capacity for full conversion under mild reaction conditions.
To estimate the oxidation mechanism at the oil–water interfaces, we compared the final products with different initial toluene concentrations (Fig. 2g). As the initial toluene concentration increased from 0.01 M to 10.00 M, both conversion and selectivity towards benzaldehyde were decreased. Moreover, various products, including benzyl alcohol, cresol, and bibenzyl emerged (Table 1 and Supplementary Fig. 9). Within the possible products, the presence of bibenzyl, which is commonly formed by the recombination of two benzyl radicals45, implies that the benzyl radical would be an intermediate of the on-water toluene oxidation. We observed the stable C7H7+ (m/z 91.0549), generated by the loss of one electron from the benzyl radical14, via microdroplet mass spectrometry operating in positive mode (Supplementary Fig. 10). Reduced conversion and selectivity with decreasing dissolved oxygen concentration (Supplementary Table 3) indicate that the produced benzyl radicals seem to primarily interact with oxygen molecules and are directly oxidized to benzaldehyde46, as opposed to forming benzyl alcohol by OH·46,47. Increased OH· concentration with dissolved oxygen (Supplementary Fig. 11) may assist benzaldehyde production.
Typically, toluene reaction with OH· can occur either through OH-addition or H-abstraction46,47. In bulk reactions, the OH-addition dominates overall reactions43,46,47,48 (~90%), while the H-abstraction contributes the remaining 10%, because the OH-addition is energetically more favorable than the H-abstraction (Table 1, entry 10). In our system, however, the formation of benzyl radical and further oxidized product benzaldehyde was predominantly observed, indicating that H-abstraction is the major reaction pathway and OH· reaction with the benzene ring is suppressed at the 2D microenvironment of oil–water interfaces.
Origin of the enhanced oxidation selectivity on water
Oil–water interfaces provide a heterogeneous microenvironment where interesting phenomena can emerge. At the oil–water interfaces, for instance, water molecules form an uneven number of hydrogen bonds and protrude free OH groups into the oil phase1,2,3, which can actively participate in the formation of hydrogen bonds as hydrogen bond donors. Among the members of the hydrogen bond family is the π–hydrogen bond. Benzene rings of aromatic compounds can function as hydrogen bond acceptors to form π–hydrogen bonds. Such interactions have been well characterized in a variety of states, including the cold clusters49, the interiors of proteins50, liquid waters51, and even the oil–water interfaces52.
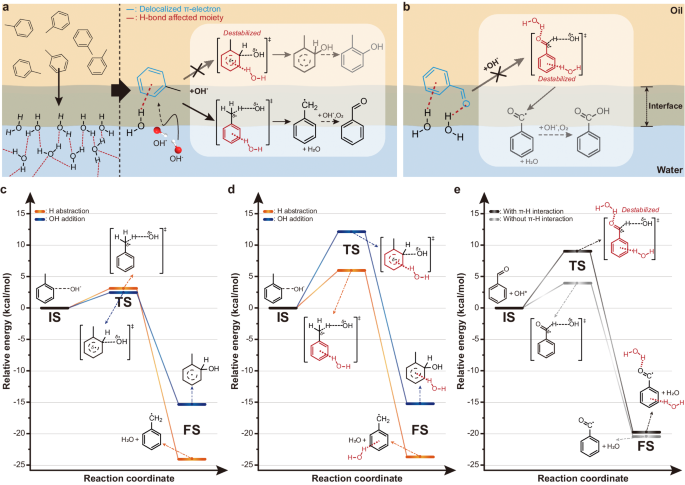
During the toluene oxidation at on water interfaces, where toluene reacts with OH·, the π–hydrogen bond between toluene and water may have a significant impact on reaction pathways. Toluene typically undergoes OH-addition by OH· rather than H-abstraction because the former has a lower transition state energy46,47. However, activation of C(sp3)–H bond by H-abstraction is expected when the exposed free OH group of water forms π–hydrogen bond with the benzene ring of toluene (Fig. 3a). Toluene donates π electrons to the free OH groups of water upon the formation of the π-hydrogen bond. The lack of π electrons may destabilize the transition state of OH-addition in which the benzene ring of toluene reacts with OH·. In contrast, the deficiency of π electrons may not strongly influence on the transition state of the H-abstraction where the methyl groups of toluene react with OH· to activate C(sp3)–H bonds. Thus, the benzyl radical generated by H-abstraction would rapidly react with an oxygen molecule to form benzaldehyde. Furthermore, as depicted in Fig. 3b, the synthesized benzaldehyde on water might also form hydrogen bonds with the free OH groups at the oil–water interfaces to suppress autoxidation and the formation of benzoic acid. While benzaldehyde is easily oxidized to benzoic acid on exposure to oxygen or OH·39, it has been reported that hydrogen-bonding environments inhibit further oxidation53.
a Schematic showing the selective C(sp3)-H activation of toluene on water. Upon interaction with OH∙, toluene forms π–hydrogen bond with the exposed free OH groups at the oil–water interface. The π–hydrogen bond destabilizes the transition state of the OH-addition, while the H-abstraction remains unchanged, resulting in the effective activation of C(sp3)–H bonds to synthesize benzaldehyde. As a representative OH-addition reaction, ortho-adduct was depicted. b Schematic illustration of benzaldehyde reaction with OH∙ at the phase boundary. Hydrogen bonds formed between the produced benzaldehyde and the free OH groups on water prevent it from being further oxidized to benzoic acid. c, d Potential energy profile of toluene-OH∙ reaction in bulk oil (c), and at the oil–water interfaces (d). The ortho-addition was used to illustrate an exemplary OH-addition reaction. e Comparison of the enthalpy profile caused by the reaction of benzaldehyde with OH∙ in bulk oil and at the oil–water interfaces. IS, TS, and FS in each reaction mechanism represent the initial state, transition state, and final state, respectively.
In addition, the decrease in selectivity with increasing initial toluene concentration in Fig. 2g may point out the importance of the ratio of exposed free OH groups to aromatic compounds at the oil–water interfaces. If there are enough protruding free OH groups to form π–hydrogen bonds with the benzene ring, it could block the OH-addition and activate the C(sp3)–H bonds. However, the increased amount of toluene leads to a deficiency of π–hydrogen and the onset of the OH-addition. Nonetheless, in our system, 10 M toluene (100% v/v) still showed a selectivity higher than 90% for C(sp3)–H bond activation. Reduced selectivity for benzaldehyde may originate from increased concentration of the benzyl radical intermediates, which can be converted to benzyl alcohol by OH· and to bibenzyl by the recombination of two benzyl radicals.
To investigate the interfacial behavior of toluene at the oil–water interface, we employed density functional theory (DFT) calculations (Fig. 3c–e). We theoretically examined enthalpy profiles of H-abstraction and OH-addition in bulk oil and at the oil–water interfaces. The ortho-adduct reaction, which is the most energetically favorable OH-addition reaction46,47, was selected for comparison. Note that model systems and calculation details are provided in the Simulation details section in Methods. In case of the toluene in the bulk oil (Fig. 3c and Supplementary Fig. 12), the activation energy of the H-abstraction reaction of toluene was greater than that of the OH-addition reaction. On the other hand, the intermediate formed by the H-abstraction reaction was thermodynamically favorable than that formed by the OH-addition reaction. Since there exists a crossover between activation energy and heat of reaction in reaction coordinate, the highly selective formation of benzaldehyde might not be observable. In case of the oil–water interface (Fig. 3d and Supplementary Fig. 13), the activation energy for the OH-addition reaction of toluene significantly increased (i.e., ~9.7 kcal/mol), compared to that in the bulk oil. Also, despite a slight increase (i.e., ~2.9 kcal/mol) of the activation energy of the H-abstraction reaction, the exothermic heat of the reaction became larger in the H-abstraction reaction compared to the OH-addition reaction. Therefore, it was expected that the selective formation of benzaldehyde from the H-abstraction reaction could be higher at the oil–water interface.
To understand the different results, the interaction between toluene and water from the molecular dynamic (MD) simulation, of which configuration was brought to perform the DFT calculation, was investigated. We observed that there exist considerable π-hydrogen interactions (see the initial state in Supplementary Fig. 13a). In addition, a molecular electrostatic potential map analysis was conducted for the toluene interacting with water or oil (Supplementary Fig. 14). According to the electronic potential map, the electronic structure of toluene interacting with oil molecules showed a marginal difference from the pure toluene. However, toluene interacting with water molecules through π–hydrogen interaction showed electronic deficiency due to the electron transfer from toluene to water. Since OH· prefers electron-rich state for its attachment; it tends to act as an electron-withdrawing group when oxidizing agent is around54, the OH-addition reaction becomes more challenging in the toluene interacting with water molecules. As a result, the alteration of the electronic structure driven by the π–hydrogen interaction at the oil–water interface could prevent the OH-addition reaction, allowing the selective formation of benzaldehyde. In addition, the peroxidation reaction by the H-abstraction reaction was investigated with benzaldehyde (Fig. 3e and Supplementary Fig. 15). At the oil–water interfaces, benzaldehyde exhibited two distinct forms of hydrogen bonds: π–hydrogen bonds and hydrogen bonds forming between the carbonyl group and water. It was theoretically observed that if there were no hydrogen-bonding interactions, the benzoyl radical might be formed. However, since the oil–water interfaces are majorly present, hydrogen-bonding interactions prevailed (see initial state in Supplementary Fig. 15b). Then, the H-abstraction reaction could be hindered as seen from greater activation energy (i.e., ~9.0 kcal/mol) with the presence of hydrogen bonds than without it. To this end, the oil–water interface is a good environment to selectively oxidize the toluene by inducing C(sp3)-H bond activation and to prevent the peroxidation of benzaldehyde, mostly by π–hydrogen interactions.
Impact of \({{{{\boldsymbol{\pi }}}}}\)–hydrogen bond strength at the oil–water interface on selective oxidation of water
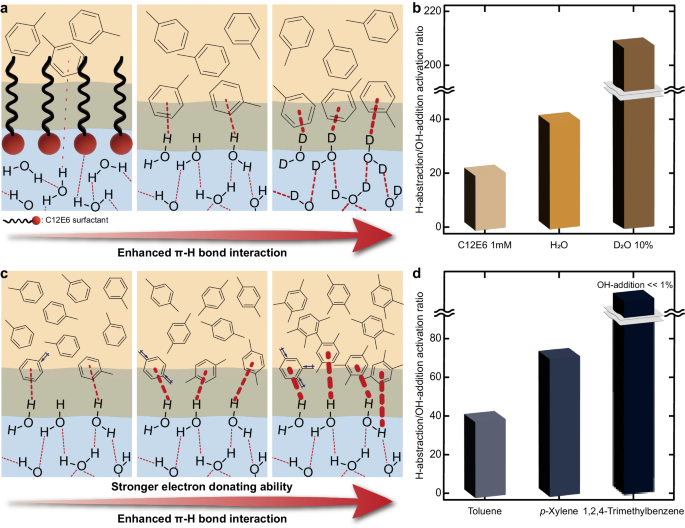
To demonstrate the effect of the π-hydrogen bond on water, we further evaluated the oxidation of 2 M toluene by modifying the oil–water interfacial properties (Fig. 4a). With the addition of 1 mM hexaethylene glycol monododecyl ether (C12E6) surfactant to the water phase, the ratio of H-abstraction to OH-addition dropped from 41 to 21 (Fig. 4b, see Supplementary Fig. 16a and Supplementary Table 4 for detailed information). Since C12E6 surfactant has a strong affinity for the oil–water interfaces55, which can efficiently impede molecular interactions across the interface56, the declined selectivity may have been caused by the loss of π–hydrogen bonds to facilitate OH-addition. On the other hand, when 10% (v/v) of the water phase was replaced with deuterated water (D2O), the selectivity for C(sp3)–H activation was boosted. D2O could serve as a stronger hydrogen bond donor57; therefore, it may form a robust π–hydrogen bond with the benzene ring of toluene to suppress the OH-addition and strongly activate C(sp3)–H bonds.
a Schematic depiction of the different interfacial interactions resulting from the distinct microenvironments. b Activation ratio of H-abstraction to OH-addition in various reaction environments. c Schematic showing the influence of electron-donating properties on the π–hydrogen bond strength at the oil–water interfaces. d Activation ratio of H-abstraction to OH-addition of three different aromatic compounds. Reaction conditions: 1 atm oxygen, 25 oC (298 K), water-to-oil ratio of 10:1 (v/v), 2 M aromatics compounds, 10 h reaction.
In addition, the strength of the π-hydrogen bond was adjusted by introducing electron-donating methyl groups to the benzene ring of toluene (Fig. 4c). As the electron-donating character of substituents increases, the strength of the π-hydrogen bond could increase58. 2 M of toluene, p-xylene, and 1,2,4-trimethylbenzene were dissolved in hexadecane and emulsified by irradiating ultrasound to 10:1 (v/v) mixtures of water and hexadecane solutions. The ratio of H-abstraction to OH-addition improved from 41 to 74 when toluene was substituted with p-xylene (Fig. 4d, see Supplementary Fig. 16b and Supplementary Table 4 for detailed information). In addition, 1,2,4-trimethylbenzene, which has the foremost electron-donating property and thus the most powerful π–hydrogen bonds, exhibited a dramatically increased selectivity for the C(sp3)–H activation, or H-abstraction (OH-addition <1%). Numerous correlations between π–hydrogen bonds and oxidation selectivity demonstrate that the interaction between aromatic compounds and interfacial water molecules was the crucial factor for the selective oxidation of toluene on water environments.
Generality of selective C(sp3)–H bond activation in aromatic compounds on water interfaces
To expand our strategy for various oil–water interfaces, we first estimated toluene oxidation by varying chain lengths of hydrocarbon oils (Supplementary Table 5). Regardless of the chain length of different hydrocarbons, the generated S/V ratios were comparable to those of hexadecane, which may have been caused by the reaction system being dominated by ultrasonic energy rather than surface tension30. All of them showed nearly perfect selectivity for benzaldehyde production at an initial toluene concentration of 0.01 M, suggesting the significance of the 2D interfacial microenvironment irrespective of oil type. However, the conversion rate strongly decreased as hydrocarbon length decreased; this trend may be correlated with the production rate of H2O2, or OH· (Supplementary Fig. 3b).
We further evaluated the versatility of selective oxidation of various aromatic compounds, including benzene, o-xylene, m-xylene, p-xylene, and 1,2,4-methylbenzene (Supplementary Figs. 17–21 and Supplementary Table 6). For 0.01 M benzene, which does not contain an additional methyl group, nothing was produced after 10 h reaction on water. However, about 1% of benzene was converted to phenol when the oil phase was composed of 10 M benzene. This also demonstrates the crucial role of the ratio between exposed free OH groups and aromatic compounds. Similarly, 0.01 M o-xylene, m-xylene, p-xylene, and 1,2,4-methylbenzene were always oxidized to aldehyde products, whereas OH-addition was observed for 10 M o-xylene, m-xylene, p-xylene, and 1,2,4-methylbenzene.