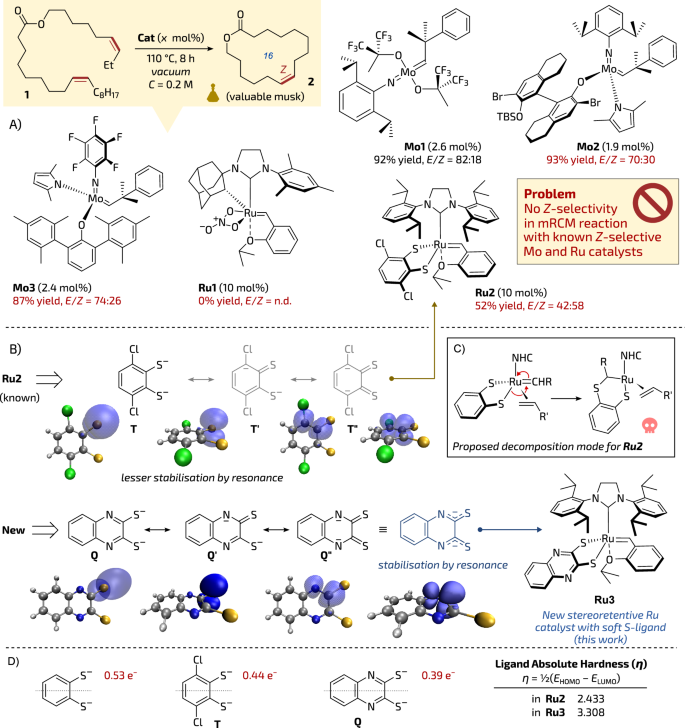
Because a number of important musk molecules possess Z-configured double bonds—profiting from well-established and recently commercially available Z-selective molybdenum21,22 and ruthenium22,23 catalysts—we decided to test whether the above high-temperature polymerisation-depolymerisation process can be conducted in a stereoselective fashion allowing the synthesis of musk macrocycles as valuable Z-isomers (Fig. 1A). As a model transformation in our study we selected mRCM of diene 1 leading to 16-membered lactone 2—a macrocyclic musk that was previously obtained under classical high-dilution conditions17,24,25,26,27. Initial tests showed that diene 1 placed under vacuum in a distillation apparatus and heated to 110 °C in the presence of 2.6 mol% of non-Z-selective molybdenum catalyst Mo1 (recently conveniently available as paraffin pellets28) in PAO-6 (a type of synthetic paraffin oil used in car engines) used as a diluent, produced a distillate containing (Z/E)-2 (92% of yield) as a 18:82 mixture of Z/E-isomers (Fig. 1A). Encouraged by this result, we decided to test the Z-selective molybdenum monoaryloxide pyrrolide (MAP) catalyst Mo2 (also conveniently available as paraffin pellets)29, in hope of obtaining selectively (Z)-2 using the same reactive distillation method. Using the previously elaborated conditions (PAO-6, vacuum, 110 °C, 8 h), we were pleased to see that lactone 2 was produced in a high isolated yield of 93%. Unfortunately, despite the use of the Z-selective catalyst, the E-cycloalkene was again formed with a significant preference (Z/E = 30:70), which is rather typical to standard, not Z-selective catalysts, such as Mo1 and not to Mo229.
A Deficiency of selectivity in high-temperature reactive distillation mRCM exhibited by known Z-selective catalysts. Yields of isolated pure products. B Key resonance structures of known thiocatechol (T) and proposed (Q) dithiolate anionic ligands. C Reported decomposition mode for stereoretentive catalysts via 1,2-sulphide shift (ref. 38). D Charges on sulphur (anionic species) in selected ligands and absolute hardness (η) calculated based on frontier molecular orbitals energies (at the M06L/def2-TZVP(PCM(THF))∼SDD//BP86-D3/def2-SVP∼SDD level of theory) of the ligands in Ru2 and Ru3. n.d. not determined. The skull icon is from Font Awesome’, Copyright (c) 2024 Fonticons, Inc. (https://fontawesome.com).
Similarly, another commercially available Z-selective catalyst, Mo3, led in high-concentration mRCM distillation to macrocycle 2 in a very satisfactory yield of 87%, albeit again with a rather disappointing Z-stereoselection (Z/E = 26:74). Given the highly unsatisfactory selectivity of commercial Z-selective molybdenum complexes, we opted to test the ruthenium family of Z-selective catalysts.
Unfortunately, the commercially available Z-selective complex Ru130,31 failed to produce even traces of macrocycle 2, which can be attributed to its instability under reactive distillation conditions (Fig. 1A). It is worth to mention that this catalyst was very successful in mRCM under classical conditions in 3 mM solution30. Interestingly, the stereoretentive complex Ru232,33,34—initially mis-described as Z-selective catalysts32—produced a larger amount of the desired Z-isomer as compared to previously studied Z-selective molybdenum-based catalysts. Namely, the mRCM reaction of 1 conducted in PAO-6 at 110 °C with 10 mol% of Ru2 led to 2 in 52% of yield as a 58:42 Z/E mixture (Fig. 1A). However, it was reported that similar macrocyclic musk has been previously prepared using Ru2 under standard high-dilution conditions in a solution (3 mM in THF, 40 °C, 1 h) offering much higher Z-selectivity level (up to 95:5 Z/E)26,35. Despite the non-perfect selectivity exhibited by Ru2 (58:42 Z/E) in the high-concentration mRCM reaction we have seen this result as the most promising, and therefore decided to focus on the Z-stereoretentive class of Ru olefin metathesis catalysts32,33,36,37. Apparently, rather harsh conditions of high-temperature reactive distillation require significantly more stable catalysts to allow high stereocontrol in the metathesis step. The mechanism of stereoretentive catalysts degradation in the presence of terminal olefins via 1,2-sulphide shift was studied in detail38. According to Hoveyda et al., the reactive alkylidene-ruthenium species formed due to the Chauvin mechanism39 are attacked by the sulphide anion of the dichlorocatecholthiolate ligand placed opposite to the NHC ligand (trans effect). This 1,2-sulphide shift leads to the generation of catalytically inactive S-ruthenium complex (Fig. 1C)38. Logically, we reasoned that by replacing the strong nucleophilic thiocatecholate dianion T (such as in Ru2) with a weaker nucleophile stabilised by resonance, like in Wang’s paper40, may perhaps render such designed disulphide ligand less prone to trigger catalysts decomposition via 1,2-sulphide shift mechanism. At the same time, we were afraid that the higher stability can come at the cost of lower catalyst activity and selectivity, as it was reported before40.
Based on basic textbook knowledge41 and supported by Density Functional Theory (DFT) calculations, we selected a 2,3-dithioquinaxoline as a precursor for such resonance stabilised ligand (Q), leading to a likely thermally more stable complex Ru3 (Fig. 1B). In fact, nucleophilicity of the sulphide atoms is seen reduced when looking at the corresponding natural charges of each ligand (from –0.44 e− in T to –0.39 e− in Q). This can be associated with the increased resonance of the new system, already demonstrated in the NBO analysis (see Table S9 in Supplementary information for further details). As a result, the delocalisation of the charge onto the additional aromatic ring of dithioquinoxaline, absolute hardness of the ligand (η), that is half of the HOMO-LUMO gap42, increases by 0.035 eV for Ru3 with respect to Ru2, particularly for the further stabilisation of the HOMO orbital (0.078 eV) compared to the one of the LUMO (0.043; Fig. 1D).
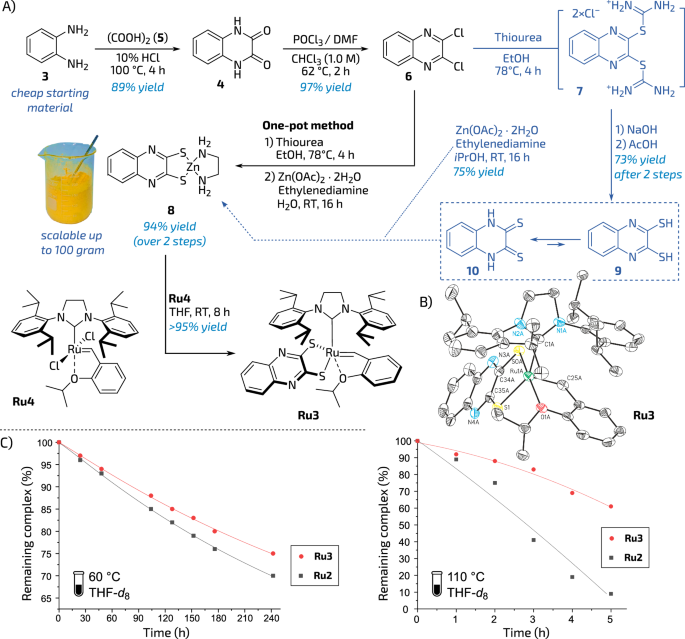
Ligand and catalysts preparation
Based on these considerations, we attempted to prepare the required ligand precursor (Fig. 2A). As a result, a straightforward route has been developed, in which a cheap starting material, benzene-1,2-diamine (3) was converted to 1,4-dihydroquinoxaline-2,3-dione (4) by an acid-catalysed condensation with oxalic acid (5). The obtained dione 4 was further transformed into 2,3-dichloroquinoxaline (6) using phosphorus(V) oxychloride. Subsequent interaction of 6 with excess of thiourea led to the formation of non-isolated intermediate (7) that was reacted in situ with zinc acetate (Zn(OAc)2×H2O) in the presence of ethylenediamine. The resulting zinc salt 8 was isolated by simple filtration as a canary-yellow solid with excellent yield (90%; see Fig. 2A). It is worth mentioning that such a simple three-step protocol does not require any special conditions and is perfectly suitable to enable large-scale synthesis (tested up to 100 g; for details, see Fig. S1, section 2.2 in supplementary information). Interestingly, by simple hydrolysing of intermediate 7, it was possible to isolate quinoxaline-2,3-dithiol 9 (more precisely—its tautomeric form—quinoxaline-2,3-dithione, 10). (Predominance of dithione (=S) form 10 over dithiol (–SH) 9 in a tautomeric equilibrium43 visibly differentiates this compound from the thiocatechol ligand precursor). The dithioquinoxaline zinc salt 8 was then reacted in THF (RT, 8 h) with the commercially available ruthenium precursor Ru4 leading to a complex Ru3 (Fig. 2A). Subsequent filtration through Celite® and concentration under reduced pressure, gave the corresponding ruthenium complex Ru3 as a brownish solid in 95% yield (note that this step requires strict exclusion of air). Such an obtained catalyst in a solid form can be stored under an argon atmosphere for almost an undefined period of time; however, we noted that in air, ca. 5% of solid Ru3 is decomposed over 24 h (for details, see Fig. S3, section 2.31 in supplementary information). To evaluate Ru3 stability in a solution, a quantitative NMR thermal stability study has been performed in THF-d8 (C = 1.0 mM) at 60 and 110 °C under argon. As a result of this measurement, it was found that in a solution Ru3 exhibits slightly more pronounced thermal stability as compared to Ru2 (Fig. 2B, for details, see Tables S1, S2, section 2.32 in supplementary information).
A Preparation of ligand precursor (9) and catalyst Ru3. Yields of isolated pure products. B Solid-state crystallographic structure of Ru3 (25% probability ellipsoids, hydrogen atoms omitted for clarity). C Stability comparison of Ru2 and Ru3 in THF-d8 solution (C = 0.1 mM) at 60 and 110 °C under argon (anthracene was used as an internal standard). Lines are visual aids only. The test tube icon is from SVG Repo, Items And Tools Icooon Mono Vectors Collection, Public Domain License (https://www.svgrepo.com).
Crystals for XRD measurement were obtained by placing a DCM solution of the Ru3 complex in an NMR tube, then layering n-hexane and allowing the solvents to slowly diffuse. Two molecules of the catalyst were found in the asymmetric cell unit. The measurement showed similar values of the most important geometric parameters as compared with those from the reported XRD of a thiocatecholate-complex (Table 1)38. The distance between the NHC carbon and Ru in Ru3 was 2.077 and 2.107 Å (two molecules in the asymmetric cell unit). The distances between sulphur atoms and ruthenium were slightly shorter and amounted to 2.3190 and 2.3199 Å for the Ru-S1 bond, and 2.2840 and 2.2818 Å for the Ru-S0 bond. The O-Ru-S0A angle was 169.44° and 168.59°. The key CNHC-Ru-S1 angle is amounted to 152.21° and 153.76°.
Tests in reactive distillation conditions
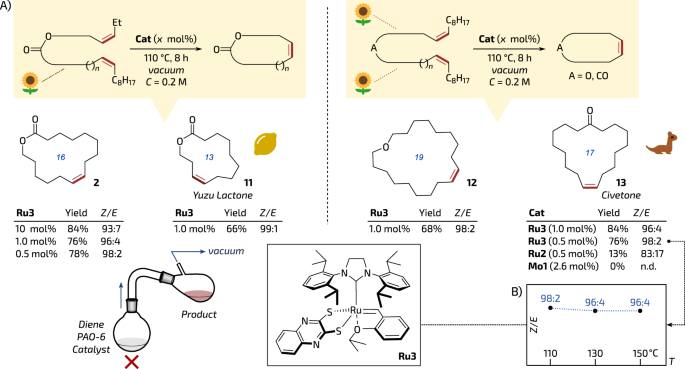
Having complex Ru3 in hand, we attempted to test it in the high-concentration Z-selective mRCM reaction at high temperatures. To do so, we used the same model diene 1 and conditions as previously (PAO-6, 200 mM, vacuum, 110 °C, 8 h; see Fig. 1A). Surprisingly, Ru3 performed in the title reactive distillation very well, giving the musk product 2 in 84% isolated yield and—this time—with very good Z-selectivity (93:7 Z/E, Fig. 3A), which is in striking contrast to Ru2 providing—under the same conditions—2 in 52% yield and with much less stereoselection (Z/E = 58:42, compare in Fig. 1A). In particular, it was even possible to decrease Ru3 loading to as low as 0.5 mol% without reducing the yield of the musk product (78%) while maintaining excellent Z-selectivity (98:2 E/Z, Fig. 3A).
A Examples of bio-based (partially or fully derived from oleic acid, which is symbolised by a sunflower icon) macrocycles obtained in reactive vacuum distillation at high concentration. Yields of isolated pure products. B (in inset) Ru3 Z-selectivity proven up to 150 °C. The citrus icon is from Font Awesome’, Copyright (c) 2024 Fonticons, Inc. (https://fontawesome.com). The sunflower icon is from SVG Repo, Fxemoji Emojis Collection, Apache License and the civeta icon is from SVG Repo, Animals 30 Collection, CC0 License (https://www.svgrepo.com). The distillation set is adapted from A. Sytniczuk, M. Milewski, M. Dąbrowski, K. Grela and A. Kajetanowicz, Green Chem., 2023, 25, 2299–2304, DOI: 10.1039/D2GC02988J.
Next, we attempted to obtain a natural product—Yuzu lactone (Z)-11, a camphor & minty-smelling 13-membered macrocycle isolated from the flesh and peel of the Japanese citrus tree Citrus junos Tanaka (Fig. 3A)44. Interestingly, this Z-configured unsaturated lactone has been previously obtained using an indirect diyne metathesis/semihydrogenation sequence45,46. More recently, Yuzu lactone was stereoselectively prepared by a direct RCM reaction catalysed by Mo-based MAP complexes; leading to 11 in 46–49% of yield with 69:31 to 73:27 Z/E selectivity (reactions were conducted in a high-dilution regime: 5 mM)47. (For comparison, non-Z-selective catalyst, Mo1, was reported to give in a solution product 11 in 30% yield as 17:83 Z/E mixture.)47 Ruthenium Z-selective complex Ru1 gave in this reaction (7.5% mol%, 3 mM in DCE, 24 h) 40% of yield and Z/E = 86:1430. On the other hand, stereoretentive Ru complex, Ru2 (used in 6 mol% loading) in a diluted DCM solution (3 mM) gave 11 in 68% yield and high selectivity (>95% Z)26. In this context we were pleased to see that only 1 mol% of Ru3 under the reactive distillation conditions (110 °C, PAO-6, 200 mM) led to Yuzu lactone 11 in isolated yield of 66% and high Z-selectivity of 99:1 (Fig. 3A).
To further explore the scope of our stereoretentive catalyst, another experiment was conducted in the presence of 1 mol% of Ru3, leading to the isolation of 19-membered macrocyclic ether 12 with satisfactory yield (68%) and similarly high Z-selectivity (≥98:2 Z/E, Fig. 3A).
Another example of Ru3 being favoured for the discussed transformation is mRCM leading to Civetone (13)—a naturally occurring animal musk isolated from the glands located near the rectum of the African civet (Civettictis civetta) and the large Indian civet (Viverra zibetha). We were pleased to see that with a loading as low as 1.0–0.5 mol% our catalyst gave macrocyclic ketone (Z)-13 in 84–76% isolated yield with perfect stereoselectivity. For comparison, the benchmark system, Ru2, under the same conditions (110 °C, 0.5 mol%) gave only 13% of 13 and worse selectivity (83:17 E/Z, Fig. 3A). Importantly, Ru3 remained impressively selective (96:4 Z/E, see insert in Fig. 3B) in mRCM production of Civetone even at an increased temperature (up to 150 °C). Finally, high-oxidation-state Mo-based alkylidene catalyst Mo1 under reactive distillation conditions failed to produce ketone 13 at all (Fig. 3B), probably because of a competing olefination reaction48,49. For comparison, under classical high-dilution conditions, 13 was obtained with 7.5 mol% of Z-selective adamantyl-complex Ru1 in 50% yield as a 68:32 Z/E mixture30 (with optimised Z-selective catalyst of the same family Civetone 13 was obtained in improved selectivity (95% of Z) but in reduced yield (36%))50. It should be noted that (Z)-Civetone (13) has also been obtained in 44% yield and in perfect E/Z selectivity of 95:5 in a continuous flow reactor using a variant of Ru1 (7.5 mol% of catalyst; 3 mM in DCE, 3 h, 70 °C)31.
Tests under classical conditions
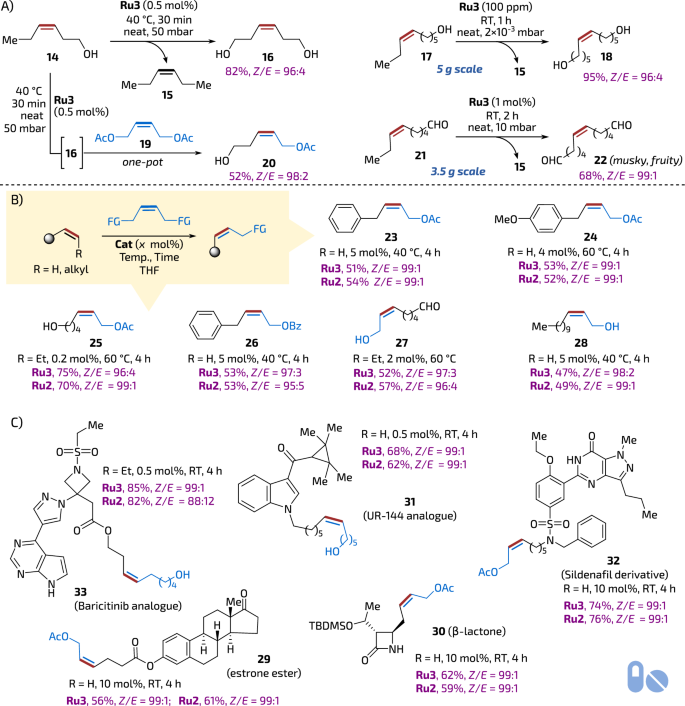
Being surprised of such high Z-selectivity levels exhibited by Ru3 that were preserved even at as high temperature as 110–150 °C during reactive distillation, we attempted a separate set of tests to verify applicability of Ru3 in reactions other than high-temperature mRCM. First, three model cross-metathesis (CM) reactions were attempted (Fig. 4A). These transformations were conducted in neat, while one of the products (3-hexene, 15) was removed under reduced pressure (Fig. 4A). Under these conditions the internal alkenes: (Z)-3-hexen-1-ol, (Z)-6-nonen-1-ol, and (Z)-6-nonenal (14, 17 and 21) were dimerised to yield valuable Z-configured functionalised products 16, 18 and 22 highly selectively (96:4 to 99:1 Z/E). Interestingly, one of the products—the bis(aldehyde) 22—was found to possess a very intense musky fruity scent. These reactions were easy to scale-up (up to 5 g) and required only a small amount of the catalyst (0.5 to 0.01 mol%) to proceed. It was also possible to conduct two different CM reactions sequentially one-pot, where the same portion of Ru3 (0.5 mol%) first dimerised substrate 14 then, in the presence of (Z)-1,4-diacetoxy-2-butene (19) promoted a CM reaction leading to bifunctional product 20 in 52% yield in two steps and with high selectivity (Z/E = 98:2, Fig. 4A).
A Z-selective CM made with Ru3 under reduced pressure without solvent (neat conditions). B Comparison of Ru2 and Ru3 in Z-selective CM under classical conditions (in THF solution). C Examples of complex polyfunctional API-derived molecules obtained in Z-selective CM. Yields of isolated pure products. API active pharmaceutical ingredients. The tablet icon is from Font Awesome’, Copyright (c) 2024 Fonticons, Inc. (https://fontawesome.com).
Next, we opted to test the activity and selectivity of Ru3 under classical conditions (in a solution), similar to those used frequently in stereoretentive catalysis34. In this context, the CM reaction of allylbenzene and (Z)-1,4-diacetoxy-2-butene (19) is commonly considered the model reaction. Although THF is the most frequently used solvent with benchmark Ru2, we decided to perform a small solvent screening study to check if the same solvent is also optimal for Ru3. To do so, EtOAc, toluene, perfluorotoluene, dimethyl carbonate (DMC), 4-methyltetrahydropyran (4-MeTHP), 2-methyltetrahydrofuran (2-MeTHF) and dichloroethane (DCE) were tested at a range of temperatures (40, 60 and 90 °C, for details, see Table S5, section 2.6.1 in supplementary information). The outcome of these studies has indicated that the choice of solvent perceptibly impacts the results of the model reaction: amongst the screened variety, THF has been found to give the best Z/E ratios and yields, rendering DMC the second choice. Thus, using Ru3 in THF at 40–60 °C substrates such as allylbenzene, 4–methoxy(allylbenzene) and (Z)-6-nonen-1-ol were reacted with 19 yielding the expected products 23, 24 and 25 in yields and selectivity fully comparable to those offered by Ru2 (Fig. 4B). In the second set of reactions, selected terminal and internal alkenes (allylbenzene, 1-dodecene and (Z)-6-nonenal) were reacted with other common cross-partners, such as (Z)-1,4-butendiol and (Z)-1,4-dibenzyloxy-2-butene in THF leading to products 26, 27 and 28. Also, in this case, activity and selectivity levels offered by Ru3 fully matched the one provided by benchmark Ru2 (Fig. 4B).
Encouraged by results observed for Ru3 under classical conditions in solution, we moved towards more sophisticated and challenging substrates. Hence, selected API (Active Pharmaceutical Ingredient) derivatives bearing various heterocyclic and Lewis basic groups (known potential chelators to Ru) were tested with Ru2 and Ru3 (Fig. 4C). It is worth highlighting that despite high structural complexity represented by the tested pharmaceutical models, starting from estrone derivative 29, trough β-lactam 30, an analogue of psychoactive cannabinoid agonist UR-14451 (31), a Sildenafil (Viagra™)52 derivative (32), the catalyst Ru3 was able to provide the expected products with always exceptional Z-selectivity (98-99%), fully matching the established stereoselective catalyst Ru2. Finally, in the case of complex polyfunctional molecule of Baricitinib53 analogue (33) quinoxaline-derived Ru2 seems to slightly outdistance Ru2 in terms of yield and selectivity (Fig. 4C).
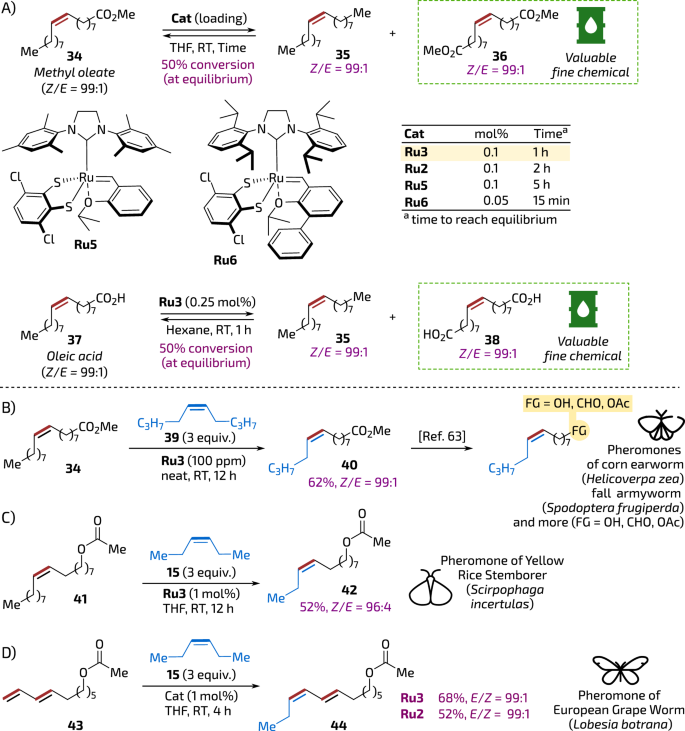
Chemical conversion of plant oils by modern catalytic methods is believed vital for developing eco-friendly routes to various building blocks and fine chemicals54,55. Therefore, we opted to test the quinoxaline-derived catalyst in Z-stereoretentive self-metathesis of oleic acid methyl ester (Z)-34, confronting it with the results obtained previously by Grubbs56 and Mauduit57. It was found that Ru3 used in 0.1 mol% loading led to 50% conversion of methyl oleate (equilibrium) in less than one hour, thus being more effective than Ru2 and Ru5 (however, the specialised, sterically activated catalyst Ru658 was reported to achieve the equilibrium in even a shorter time and at lower loading: 15 min at 0.05 mol% or 16 h at 0.01 mol%57). Importantly, products of this reactions—alkenes 35 and 36 obtained with high stereoselection (Z/E = 99:1) are known as valuable building blocks, being used inter alia in the synthesis of (Z)-Civetone (from diester 36)59,60, and various pheromones (from both 35 and 36)31,61. Catalyst Ru3 was tested in self-metathesis of oleic acid 37, and also, in this case, achieved an equilibrium with perfect Z-selectivity at only 0.25 mol% of catalyst loading within 1 h (Fig. 5A).
A Z-selective self-CM of oleic acid and its methyl ester. B–D Examples of pheromones or pheromone precursors obtained in Z-selective CM with Ru3 (for conversion of precursor 40 into pheromones, see El-Rabbat and Mangold, ref. 68). The barrel icon is from Font Awesome’, Copyright (c) 2024 Fonticons, Inc. (https://fontawesome.com). The moth icons are from SVG Repo, Bugs Insects 2, Nature 3, and Spring Icons Collections, CC0 License (https://www.svgrepo.com).
Pheromones offer a highly promising, biosphere-friendly approach to protect various crops. Unlike chemical pesticides, pheromones target only specific pest species, leaving other insects, especially pollinators (bees and wasps), unharmed2,3. Additionally, pheromones are biodegradable and used in small amounts, and therefore do not pose a threat to human health, making them ideal for modern, environmentally conscious farming62. However, their synthesis shall be possibly straightforward and fully stereoselective (as the cost of pheromone production shall be kept down to make them applied also in less developed countries. Presently, treating a one-hectare field with a regular pesticide costs between €50 and €150. The bio-control of the same surface using the pheromone costs around €250 according to reference62), as the biological action of pheromones is often related to their E/Z-geometrical constitution2,3. Therefore, as a final test for the usefulness of our dithioquinoxaline-based catalyst, we opted to prepare some well-known pheromones using Z-selective metathesis with Ru3.
First, we approached the synthesis of a precursor of sex pheromones of fall armyworm (Spodoptera frugiperda)63 oriental tobacco budworm moth (Helicoverpa assulta)64, corn earworm (Helicoverpa zea)65 and similar insects from the Noctuidae family that consume a wide variety of crops such as corn, tomato, pepper, rice, peanuts, and more. In a published method, the CM reaction of methyl oleate (34) was conducted with 1-hexene and the second-generation Hoveyda-Grubbs catalyst supported on silica. This transformation produced a rather complex mixture of products, including 1-decene, 5-tetradecene, methyl 9-decenoate and methyl 9-tetradecenoate (40)66. Obviously, due to the application of non-Z-selective catalyst, this method suffered from very low E/Z-selectivity. Therefore, Pederson and Grubbs used an alternative approach that was based on the reaction of a large excess of 1-hexene (in fact, used as a co-solvent, as a 1:1 mixture with THF) with oleyl alcohol. This process yielded a pheromone precursor—(Z)-9-tetradecenol in a 77% of yield, albeit exhibiting non-perfect Z-selectivity (Z/E = 86:14) despite the use of the Z-selective catalyst Ru1 (1 mol %)67. In turn, we decided to reinvestigate the methyl oleate route, as the precursor (Z)-40 can be later converted to a number of lepidopteran sex pheromones (Fig. 5B). As a result, we were pleased to see that 34 reacted with internal olefin 39 (3 equivalents) to lead (Z)-40 with high selectivity (Z/E = 99:1) in 62% yield, in presence of only 100 ppm of Ru3. It shall be noted that this reaction can be conveniently conducted without a solvent (in neat). Such formed (Z)-40 can be converted to valuable pheromones, such as (Z)-9-tetradecenal, (Z)-9-tetradecenol and (Z)-9-tetradecenol acetate by reduction and acetylation using reported methods (Fig. 5B)68.
Next, we attempted to do CM between oleyl acetate 41 and internal olefin 15 to obtain a pheromone of yellow rice stem borer (Scirpophaga Incertulas), a moth whose larvae causes severe damage to rice throughout the world69. The Z-selective CM reaction went in this case easily as well, leading to the formation of 4267 as almost pure Z-isomer (Z/E = 96:4, Fig. 5C). For comparison, Pederson and Grubbs, to obtain the same pheromone, used CM between oleyl alcohol and 1-butene. The optimal conditions involved slow bubbling of 1-butene into the reaction solution in the presence of 2 mol % of Ru1. Reaction with oleyl alcohol (followed by acetylation of the CM product) led, however, to the formation of pheromone 42 in modest yield (40%) and slightly reduced Z-selectivity (77% Z)67.
Finally, we decided to try CM of conjugated diene 43 with 15 to obtain sex pheromone of the European grapevine moth (Lobesia botrana)—an environment-friendly agrochemical already used to protect vineyards in Europe62. To do so, in a reaction catalysed by 1 mol% of Ru3 product 44 was formed as a single (7E,9Z)-isomer with high selectivity (Fig. 5D). Importantly, the industrially used method70, as well as the improved preparation62 let to this pheromone as (7E,9Z) and (7E,9E) 75:25 mixture of isomers.
DFT study
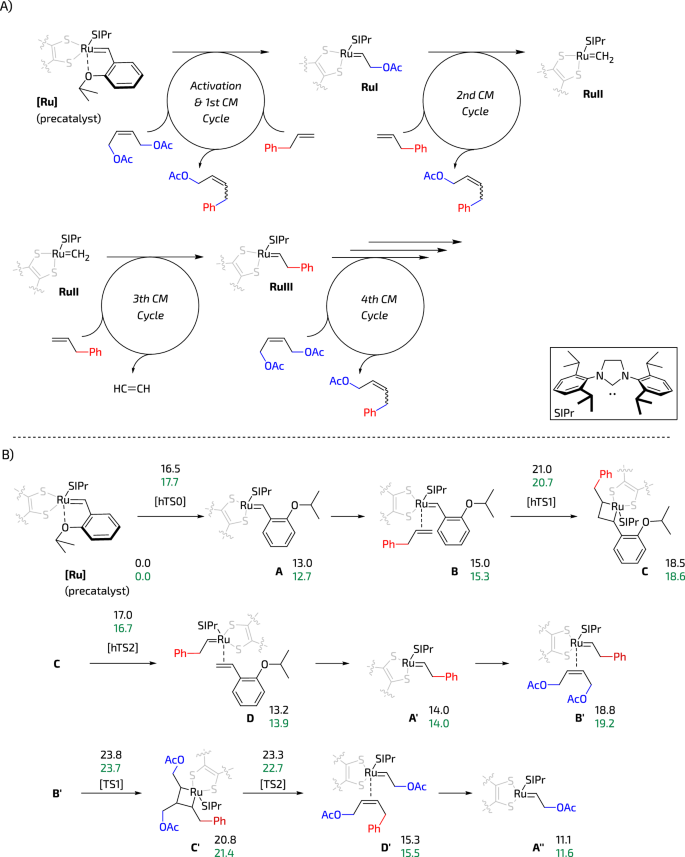
To analyse possible differences in deactivation of Ru3 and Ru2 catalysts via 1,2-sulphide shift we decided to model the CM reaction of allylbenzene and (Z)-1,4-diacetoxy-2-butene which is a popular test reaction in Z-selective metathesis (compare: Fig. 4B). When analysing the deactivation reaction paths, it is crucial to account for the various olefin combinations present. Speciation processes must be considered to accurately assess the deactivation step, as it can occur within any of the CM cycles. Figure 6A illustrates some of these cycles, and while not explicitly investigated, certain non-productive combinations were also taken into account. During the reaction precatalyst Ru2 or Ru3—after dissociation of Ru–O chelate that leads to formation of 14-e− species A—undergoes a series of 2 + 2 cycloaddition and cycloreversion reactions39 forming key propagating intermediates RuI, RuII and RuIII (Fig. 6A). During each of the consecutive catalytic cycles these intermediates can keep propagating or undergo decomposition via 1,2-sulphide shift (Fig. 1C)38. After studying the reaction sequence in silico, we noted no significant differences between Ru2 and Ru3 catalysts thermodynamics (Fig. 6B). Although the energy barriers for Ru3 appear to be slightly smaller, this reduction falls beyond the precision of DFT calculations.
A Subsequent cross-metathesis cycles were analysed. B Reaction pathway with the corresponding relative Gibbs energies (in kcal/mol) for Ru2 (in black) and Ru3 (in green). The transition states correspond to TS1 for the metallacycle formation and TS2 for the cycloreversion steps. In the initial cycle during precatalyst activation, the corresponding cases are labelled with an h- prefix, such as hTS1 and hTS2, with the chelate aperture indicated by hTS0.
Table 2 presents the thermodynamics of the 1,2-sulphide shift transition state (TS) with different alkene substituent sets during the activation and first cross-metathesis cycle. Similar to the cross-metathesis reaction discussed earlier, the disparities between both systems are, in general, small. However, two notable distinctions emerge in the following scenarios: when an ethylene molecule coordinates with a 14-e− methylidene complex (E), the 1,2-sulphide shift occurs more rapidly with the benchmark Ru2 compared to the Ru3 system. Another case where these two systems exhibit different behaviour involves the coordination intermediate comprising of methylidene and allylbenzene (F). In this instance, the kinetics favour the deactivation of Ru2 by ~5 kcal/mol.
The Z-alkene geometry is ubiquitous in a wide range of building blocks, fine chemicals, and natural products. Because well-established Mo, W, and Ru Z-selective catalysts were found to lose their selectivity at higher temperatures required for industrially interesting reactive distillation mRCM, we opted for the development of a catalyst capable of providing Z-alkenes in high selectivity, also under more harsh conditions. It was found that the new type of stabilised by resonance dithiolate ligand can provide high selectivity at temperatures up to 150 °C in highly concentrated reaction mixtures. Computational studies were employed to assess the observed enhancements conferred by these novel ligands, operating under the assumption that the primary limitation to achieving Z selectivity lies in the propensity for 1,2-sulphide displacement. While no discernible thermodynamic enhancements were identified along the Z-product route, there is evidence suggesting a reduction in the kinetics of the deactivation pathway with our ligand, particularly when considering proper speciation. Furthermore, the concurrent characterisation via parallel DFT supports the notion of a nucleophilic decrease in the sulphur nuclei within the 2,3-dithioquinoxaline ligand structure.
The above attributes distinguish the Ru3 complex from other Z-selective olefin metathesis catalysts based on Mo, W and Ru. Importantly, this unique trait did not come at the cost of limited general usability under ‘classical’ conditions. On the contrary, the new complex was found to match the activity of known stereoretentive catalysts such as Ru2 even in the case of complex polyfunctional substrates. In addition, the catalyst Ru3 can be used in the valorisation of bio-sourced alkene feedstock such as oleic acid and in the production of sex pheromones used in agriculture for pest control.